# Major Recall Alert: Tafrodol Recalled Due to Unlicensed and Addictive Opioids Containing Harmful Combinations
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued an urgent public alert regarding the illegal importation and sale of **Tafrodol**, an opioid manufactured by Aveo Pharmaceuticals. This product contains harmful, addictive combinations of **tapentadol** and **carisoprodol**, making it dangerous and unlicensed for use anywhere in the world. Consumers are urged to stop the use, purchase, or distribution of this product immediately to prevent significant health risks.
---
## Why This Recall is Important
The recall of Tafrodol is a critical measure to protect public health across Nigeria, as the opioid's composition is not only unregulated but also highly dangerous. **Tapentadol** and **carisoprodol**, the two main ingredients found in this drug, pose severe risks:
- **Harmful Interactions**: These substances combined can depress the central nervous system, leading to addiction, respiratory issues, and even death.
- **Unlicensed Worldwide**: Tafrodol is not approved for therapeutic use by any regulatory body worldwide, signifying no adequate safety or efficacy testing has been conducted.
- **High Potential for Addiction**: Tapentadol and carisoprodol have been associated with dependency and abuse, which can lead to debilitating physical and psychological side effects.
NAFDAC’s proactive measures aim to combat the spread of this illegally imported product that could worsen Nigeria's growing opioid crisis.
---
## Details of the Recall
Here’s what you need to know about the recent recall of **Tafrodol**:
- **Product Name**: Tafrodol
- **Manufacturer**: Aveo Pharmaceuticals
- **Active Ingredients**: Tapentadol and Carisoprodol
- **Reason for Recall**:
- Unlicensed and illegal opioid combination.
- Highly addictive properties.
- No safety or therapeutic approval worldwide.
- **Date Announced**: February 8, 2025
- **Images of Product**:
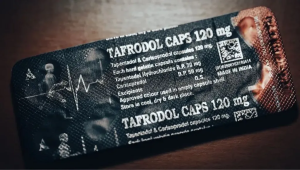

For more official details, see the complete **NAFDAC Public Alert No. 04/2025** [here](https://nafdac.gov.ng/public-alert-no-04-2025-public-alert-on-the-illegal-exportation-and-sale-of-opioids-to-west-african-countries-by-aveo-pharmaceuticals/).
---
## What You Should Do
If you suspect the presence or sale of **Tafrodol** in your area, take immediate action to ensure your safety and that of your community:
1. **Stop Usage**: Do not consume Tafrodol under any circumstances.
2. **Report Suspicious Activities**:
- Contact **NAFDAC** to report the sale or distribution of Tafrodol.
- Email: _nafdacenforcement@nafdac.gov.ng_
- Helpline: _+234 (0) 803 123 4500_
3. **Check for Substandard Medicines**: Be vigilant about the source of your medicines to avoid counterfeit or unlicensed drugs.
4. **Spread Awareness**: Inform others about the dangers of Tafrodol and the ongoing recall.
Taking swift action can help curtail this public health threat.
---
## Stay Safe – Get Instant Recall Alerts
Don’t wait for harmful medicines to affect your life. You can stay informed about dangerous recalls like Tafrodol by keeping in touch with NAFDAC’s latest public health alerts.
**Download the NAFDAC Consumer Safety App now** to receive instant notifications about drug recalls, counterfeit products, and health tips. With just one tap, you can protect yourself, your family, and your community.
[**Download the App Here**](https://nafdac.gov.ng)
---
Illegal, unlicensed drugs remain a significant health hazard across Nigeria and beyond. The swift action against Tafrodol is key to combating this crisis, but community vigilance also plays a vital role. Stay informed, report suspicious activities, and spread the word to ensure a safer tomorrow.